UFAI's Clinical Trials
The physicians at University Foot and Ankle Institute are dedicated to bringing innovative technology to our patients through clinical research and trials. These trials involve investigating leading-edge foot and ankle care, products and devices.
Our clinical trials are carefully controlled research studies that gather scientific evidence to improve the ways we prevent, diagnose or treat foot and ankle conditions. Each clinical study is led by one of our trusted medical doctors and our professional research team.
Many patients who have taken part in our clinical research trials have been helped as a result of having access to state-of-the-art care they otherwise would not have had. The first people to have been helped by medication or a medical device are often those who were part of its clinical trial.
-
Recruiting Closed
Plantar Fasciitis Pain Study
Recruitment Status:ClosedStart Date:March, 2019End Date:TBDIf you are 18-65 and have heel pain in one of your feet for the last 3 months, you may be eligible for a non-surgical treatment at no cost to you. Trial volunteers will receive study medication and procedures at no cost to them, as well as compensation for their time and travel. (No health insurance is required.)
-
Recruiting Closed
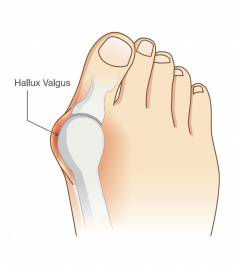
Bunion Pain Study
Recruitment Status:ClosedStart Date:November, 2018End Date:TBDThe Dystance study will look at the safety and effectiveness of a study medication compared with placebo (no active ingredients), given by injection, in adults ages 18 years or older with hallux abducto valgus (HAV), more commonly known as a bunion. What is learned from this study may help those with bunions in the future.